Research Interests
At the University of Saskatchewan
• Synchrotron X-ray Absorption Spectroscopy
• Reactive Molecular Dynamics
• Pair Distribution Function
At Oklahoma State University
I started my graduate studies synthesizing branched fluoro-oligogermanes. In the course of this project, I came across germanium cations (germyliums). I studied their immense Lewis acidity, and could apply them in hydrodefluorination (HDF) reactions. Currently, I am working on "impossible" substrates for main-group elements based HDF, and other innovative organic transformations by germanium compounds. Below, you can find summaries of some results from my projects.
• Synthesis of the Elusive Branched Fluoro-oligogermane (Ph3Ge)3GeF

Our group was successful in making (Ph3Ge)3GeX (X=Cl, Br, I) compounds (Organometallics 2011, 30, 5, 1046-1058) but making the fluoride analogue proved to be harder. After several different attempts, the key to this elusive compound was found to be taking advantage of the extreme Lewis acidity of the germylium intermediate. Typically considered to be a weakly-coordinating anion (WCA), BF4-, acted as an in situ fluoride source. Along with the anionic digermyne fluoride adduct with silver ions, made by Power et al. (J. Am. Chem. Soc. 2010, 132, 38, 13150-13151), (Ph3Ge)3GeF, are the only fully characterized compounds that have Ge-Ge and Ge-F bonds. Our attempts to isolate this germylium have been futile, but this reactive intermediate has given us insights into the potentials of germanium compounds in organic synthesis.
Publication: Organometallics 2018, 37, 12, 1852-1859, 917. [Link]
• HDF of Acid Fluorides and Organofluorines by Ph3GeH
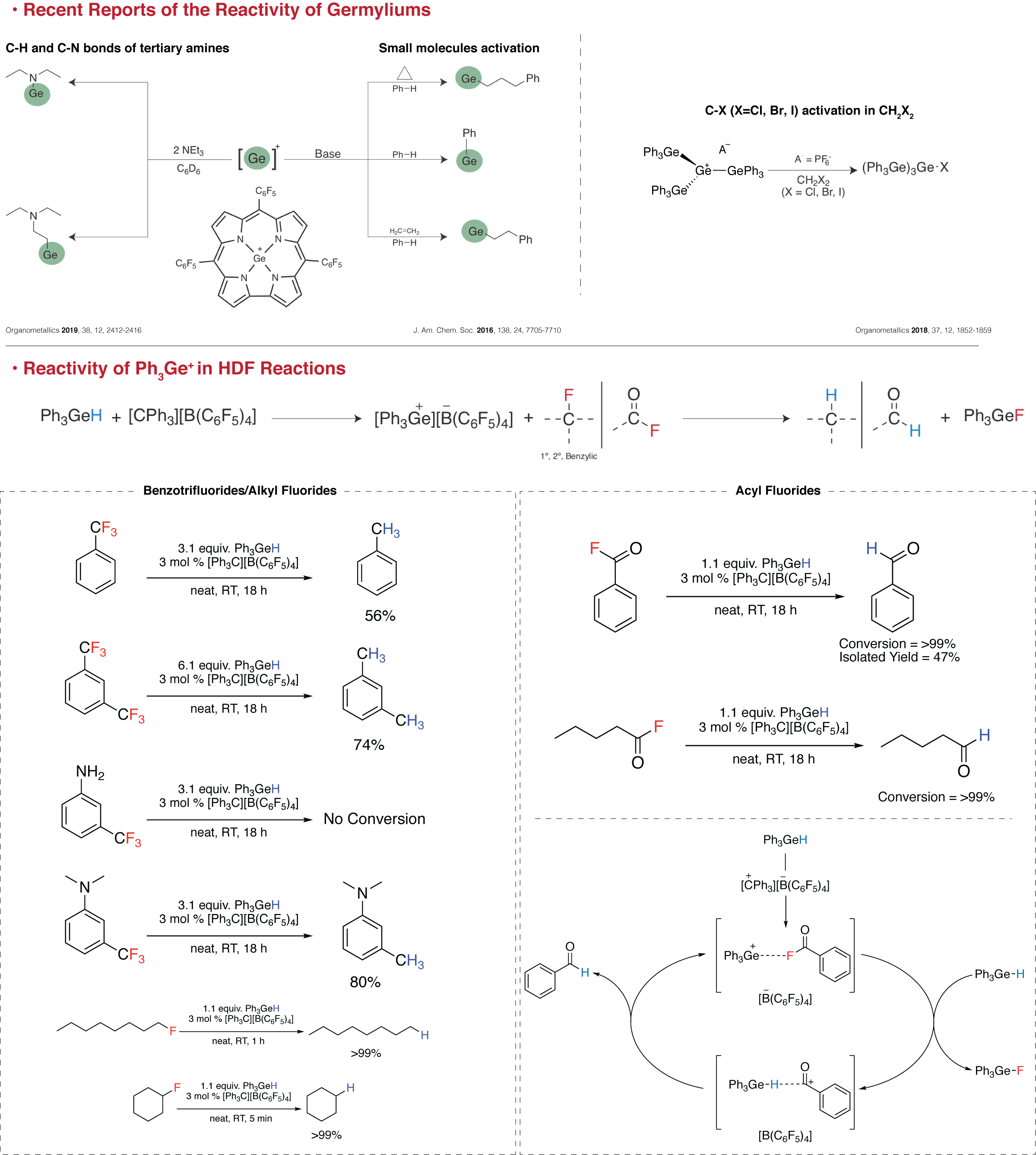
Our continuing efforts for isolating the germylium intermediate, led us to examine the capability of the simpler germylium Ph3Ge+ in C-F bond activation. In the previous project, we had realized that the branched germylium (Ph3Ge)3Ge+ is able to activate C-X bond in CH2X2 (X=Cl, Br, I). Also, there have been reports about using germyliums in C-N, C-H bonds activation, small molecules activation and unusual Friedel–Crafts reactions. We have been able to show that Ph3GeH with catalytic amounts of [CPh3][B(C6F5)4] can do HDF with acyl fluorides and organofluorines. This approach has proved to be simple and mild and can be carried out in neat conditions. Other significant features of this method are, no over-reduction of acyl fluorides or partial HDF of organofluorines happen.
My favorite part of this project is that several initial reactions were done by 10 high school students at the university of Detroit Jesuit high School (Detroit, MI). These students are included as co-authors for this paper. Also, this article was featured as the cover art for the issue in which it was published.
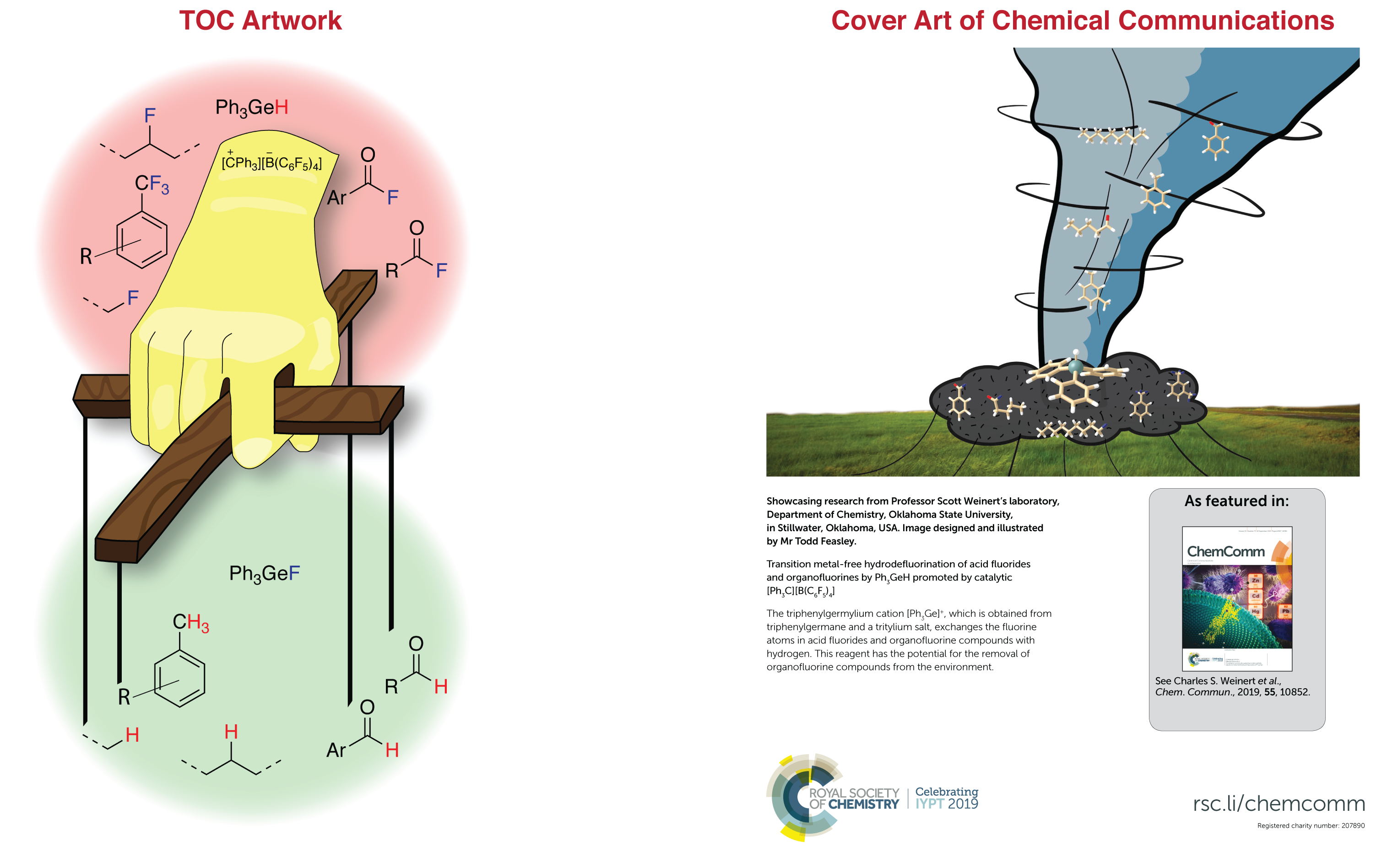
Publication: Chemical Communications 2019, 55, 73, 10852-10855. [Link]
• Direct Amidation of Acid Fluorides by Ph3GeNMe2
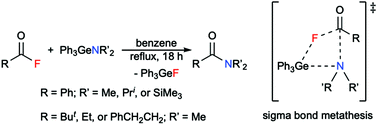
I attempted many times to get a crystal structure of different germyliums but none of them were successful. Germanium amides (or germyl amines) are compounds that our group used extensively for hydrogermylation reactions. The structure and the memory of working with germyliums led me to think that what if "germanium amides are masked germyliums" (turned out not to be true)!
I was able to show that Ph3GeNMe2 can do direct amidation of acid fluorides. This reaction is mild, clean and has quantitative yields.
Our computational studies revealed that masked germylium pathway is not energetically favorable. Instead, the reaction proceeds through a concerted, σ-bond metathesis mechanism.
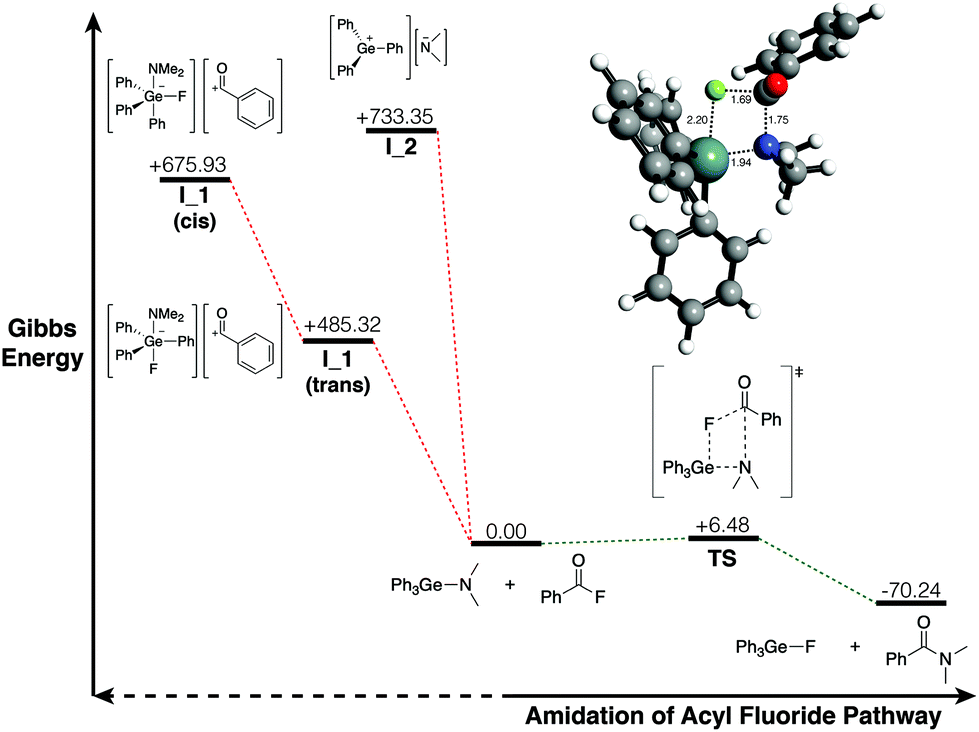
Publication: Dalton Transactions 2021, 50, 4490-4493. [Link]